- The following general reaction?will be used as an example to study the rate of reaction
D (aq) → E (aq) + F (g)?
- The rate of reaction at different concentrations of D?is measured and tabulated
D (aq) → E (aq) + F (g)?
Rate of reactions table
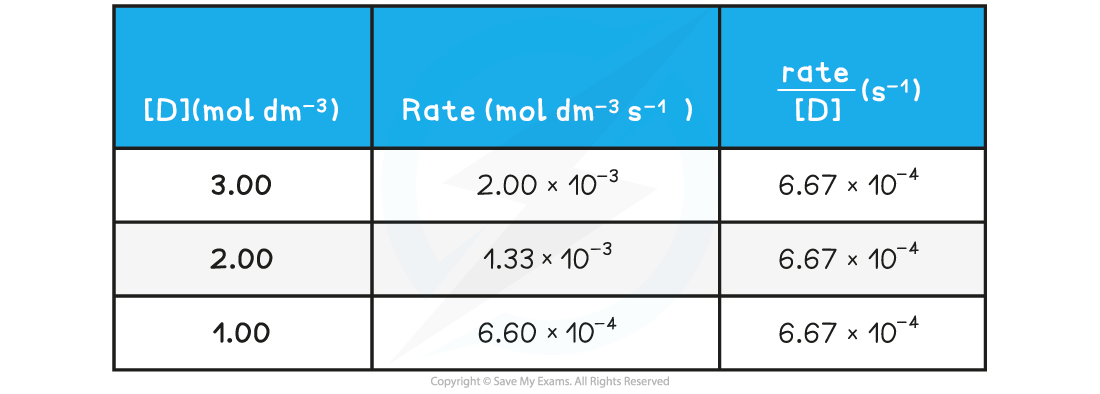
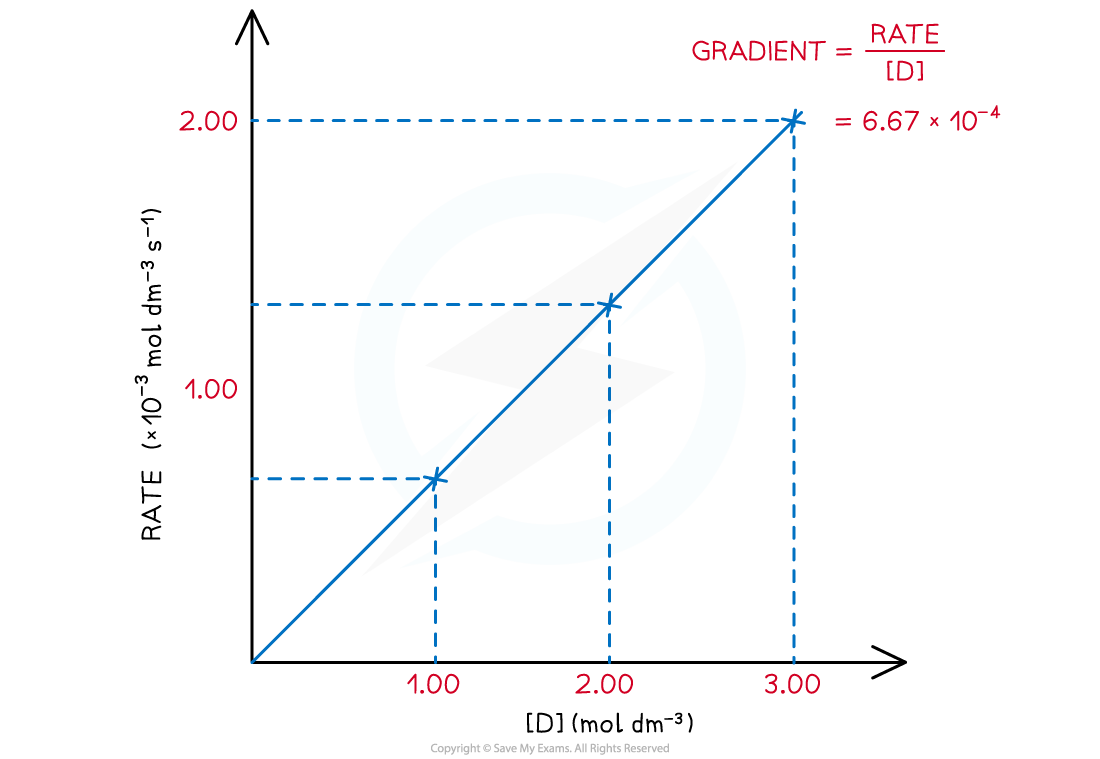
Rate of reaction over various concentrations of D
Rate ∝ [D]? ? ? ?or? ? ? ?Rate =?k[D]
A (aq) + B (aq) → C (aq) + D (g)?
Rate of reaction =?k?[A]m?[B]n
The chemical equation for the thermal decomposition of dinitrogen pentoxide is:
2N2O5?(g)?→?4NO2?(g) + O2?(g)
The rate equation for this reaction is:
Rate =?k[N2O5?(g)]
Answers
Answer 1:
Answer 2:
The following equation represents the oxidation of bromide ions in acidic solution
BrO3-?(aq) + 5Br-?(aq) + 6H+?(aq) → 3Br2?(l) + 3H2O (l)
The rate equation for this reaction is:
Rate =?k[BrO3-?(aq)][Br-?(aq)][H+?(aq)]
Answers
Answer 1:
Answer 2:
(CH3)3CBr? +? OH-? →? (CH3)3COH? +? Br-
Table to show the experimental data of the above reaction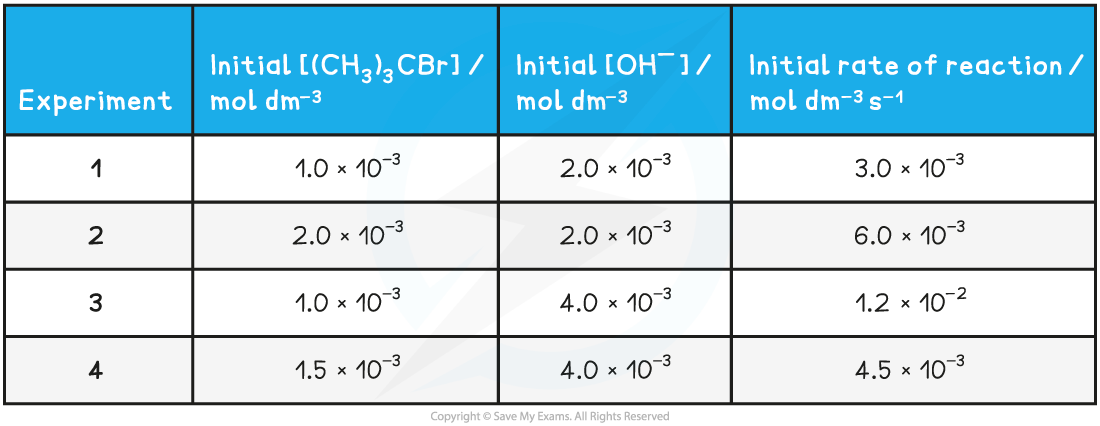
Rate =?k?[(CH3)3CBr] [OH-]2
Exam Tip
Examiners will often give concentration and rate data in standard form to test your mathematical skills!Take your time because it is easy to make a mistake - the most common one is failing to notice a factor of ten, e.g. one rate value is x10-4?while the rest are x10-3
转载自savemyexams


? 2025. All Rights Reserved. 沪ICP备2023009024号-1