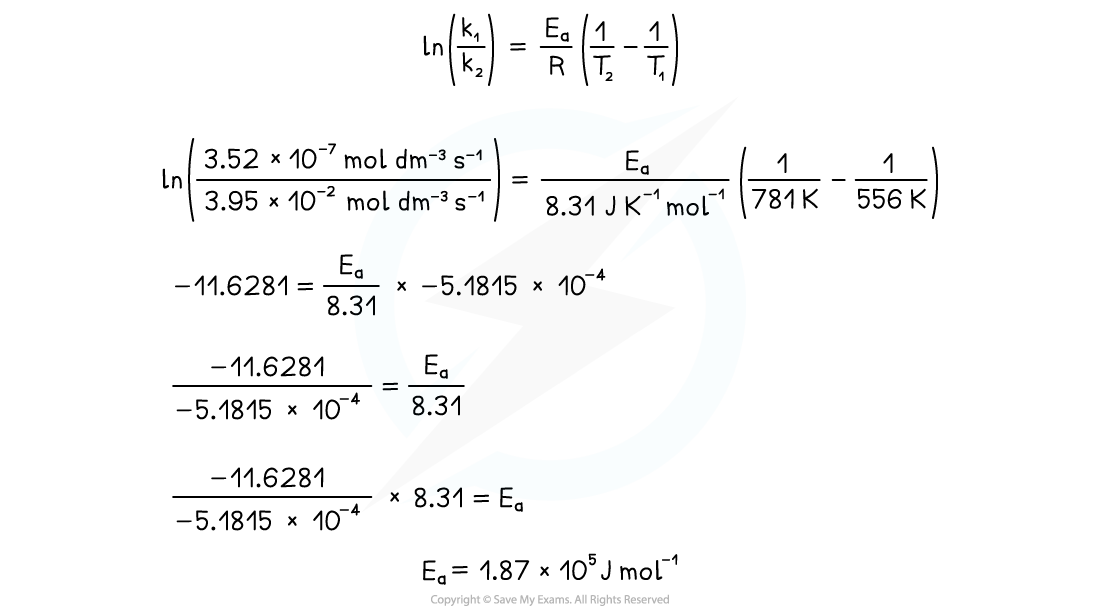Activation Energy from Rate Constants at Different Temperatures
Arrhenius Plots
- Arrhenius plots for two reactions with different activation energies can be drawn on the same graph
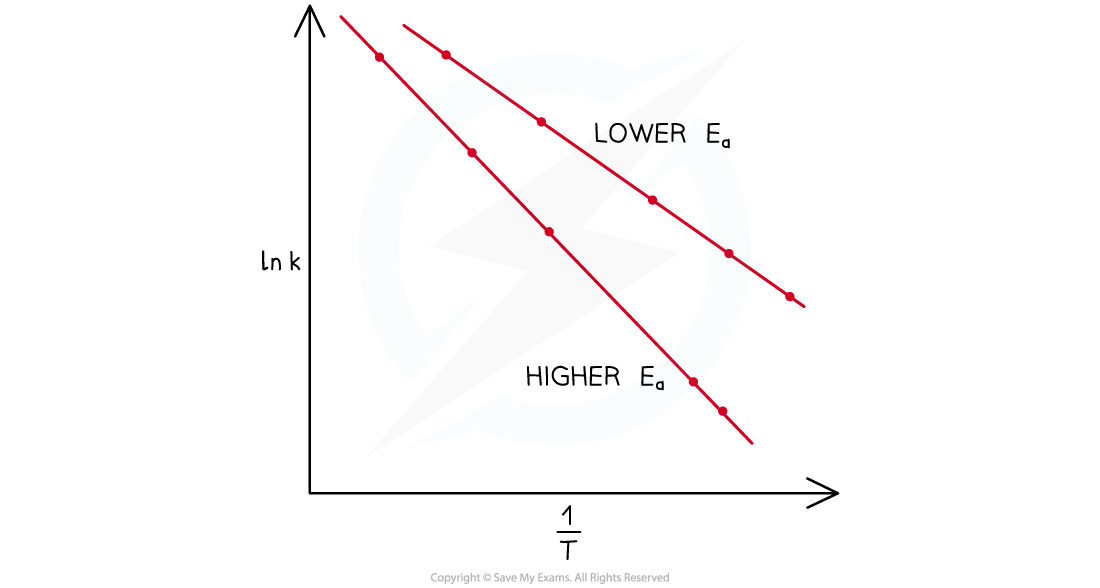
Arrhenius plots for two reactions with different activation energies
- The reaction with a steeper gradient has the higher activation energy,?Ea
- This indicates that the rate constant, and therefore rate, will change quicker with temperature changes
Calculating the Activation Energy
- The activation energy,?Ea, can be calculated using rate constant values,?k1and?k2, for two given temperatures,?T1?and?T2
- This requires the use of the following equation that is given in the data booklet:

Worked Example
Hydrogen iodide decomposes in the gas phase to form hydrogen and iodine
2HI (g) → H2?(g) + I2?(g)
At 283?oC, the rate constant is 3.52 x 10-7?mol dm-3?s-1At 508?oC, the rate constant is 3.95 x 10-2?mol dm-3?s-1Calculate the activation energy,?Ea, for the reaction
Answer
-
- Convert the temperatures from?oC to K:
- T1:283 + 273 = 556 K
- T2:508 + 273 = 781 K
- Write the appropriate Arrhenius equation from the data booklet
- Substitute the values
- Evaluate the equation to get the activation energy,?Ea
- Convert the temperatures from?oC to K: